What Is Molar Absorption Coefficient

Content
- What is tooth absorptivity?
- Units
- How to summate it?
- Direct clearance
- Graphing method
- Solved exercises
- Exercise 1
- Exercise 2
- References
The molar absorptivity it is a chemical property that indicates how much light a species tin can absorb in solution. This concept is very of import within the spectroscopic analysis of absorption of photon radiation with energies in the ultraviolet and visible range (UV-vis).
Every bit lite is composed of photons with its ain energies (or wavelengths), depending on the species or mixture analyzed, one photon tin can exist absorbed to a greater degree than another; that is, low-cal is absorbed at certain wavelengths feature of the substance.

Thus, the value of tooth absorptivity is directly proportional to the degree of assimilation of light at a given wavelength. If the species absorbs little red light, its absorptivity value will be low; whereas if there is a pronounced absorption of red low-cal, the absorptivity will have a high value.
A species that absorbs red light will reflect a green colour. If the green color is very intense and night, it means that there is a potent absorption of cerise light.
Yet, some shades of greenish may be due to the reflections of unlike ranges of yellows and dejection, which are mixed and perceived every bit turquoise, emerald, drinking glass, etc.
What is molar absorptivity?
Molar absorptivity is as well known past the following designations: specific extinction, molar attenuation coefficient, specific assimilation, or Bunsen coefficient; It has even been named in other ways, which is why it has been a source of confusion.
Merely what exactly is molar absorptivity? It is a constant that is defined in the mathematical expression of the Lamber-Beer law, and it merely indicates how much the chemical species or mixture absorbs light. Such an equation is:
A = εbc
Where A is the absorbance of the solution at a selected wavelength λ; b is the length of the jail cell where the sample to be analyzed is independent, and therefore, is the altitude that the light crosses inside the solution; c is the concentration of the absorbent species; and ε, the tooth absorptivity.
Given λ, expressed in nanometers, the value of ε remains constant; but when irresolute the values of λ, that is, when measuring absorbances with lights of other energies, ε changes, reaching either a minimum or maximum value.
If its maximum value is known, εmax, is determined at the same time λmax; that is, the low-cal that the species absorbs the most:
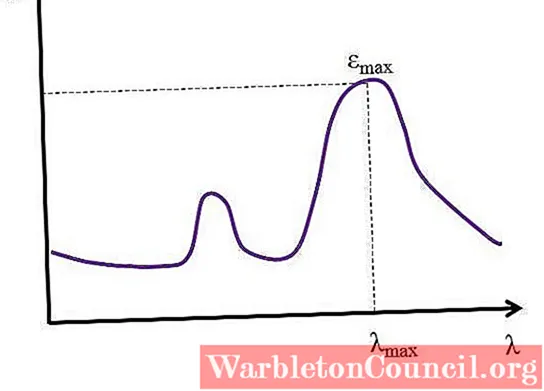
Units
What are the units of ε? To observe them, it must be known that absorbances are dimensionless values; and therefore, the multiplication of the units of b and c must cancel out.
The concentration of the absorbing species can be expressed either in g / L or mol / L, and b is commonly expressed in cm or thou (because it is the length of the cell that the lite beam passes through). Molarity is equal to mol / L, so c is besides expressed as M.
Thus, multiplying the units of b and c, we obtain: One thousand ∙ cm. What units then must ε have to make the value of A dimensionless? Those that multiplying M ∙ cm gives a value of 1 (Chiliad ∙ cm x U = ane). Solving for U, we simply obtain 1000-1∙ cm-ane, which tin also be written as: 50 ∙ mol-one∙ cm-one.
In fact, use the M units-i∙ cm-one or L ∙ mol-1∙ cm-1 streamline calculations to determine tooth absorptivity. However, it is besides frequently expressed in units of yard2/ mol or cm2/ mol.
When expressed in these units, some conversion factors must be used to modify the units of b and c.
How to calculate it?
Straight clearance
Molar absorptivity can be calculated directly by solving it in the above equation:
ε = A / bc
If the concentration of the arresting species, the length of the cell, and the absorbance obtained at a wavelength are known, ε tin be calculated. However, this fashion of calculating it returns an inaccurate and unreliable value.
Graphing method
If you await closely at the Lambert-Beer law equation, you will find that it looks similar the equation of a line (Y = aX + b). This means that if the values of A are plotted on the Y centrality, and those of c on the X centrality, a straight line must exist obtained that passes through the origin (0,0). Thus, A would be Y, X would be c, and a would equal εb.
Therefore, once the line is graphed, it is enough to have whatever two points to decide the slope, that is, a. One time this is done, and the length of the cell, b, known, it is easy to solve for the value of ε.
Dissimilar directly clearance, graphing A vs c allows the absorbance measurements to exist averaged and to reduce the experimental fault; and also, infinite lines tin can laissez passer through a single signal, and so direct clearance is non practical.
Also, experimental errors can cause a line to not pass through two, three or more points, and then in reality the line obtained subsequently applying the least squares method is used (a function that is already incorporated in calculators). All this assuming loftier linearity, and therefore, compliance with the Lamber-Beer law.
Solved exercises
Exercise ane
It is known that a solution of an organic compound with a concentration of 0.008739 M presented an absorbance of 0.6346, measured at λ = 500 nm and with a cell length 0.v cm. Calculate the tooth absorptivity of the complex at that wavelength.
From these information, ε can exist solved straight:
ε = 0.6346 / (0.5cm) (0.008739M)
145.23 M-1∙ cm-i
Practice 2
The following absorbances are measured at different concentrations of a metal complex at a wavelength of 460 nm, and with a cell of 1 cm in length:
A: 0.03010 0.1033 0.1584 0.3961 0.8093
c: i.8 ∙ ten-five 6∙10-v 9.2∙ten-5 ii.three∙ten-4 5.6∙10-4
Summate the tooth absorptivity of the complex.
In that location are a total of five points. To calculate ε information technology is necessary to graph them by placing the values of A on the Y axis, and the concentrations c on the 10 centrality. Once this is washed, the to the lowest degree squares line is adamant, and with its equation we can determine ε.
In this case, plotting the points and drawing the line with a coefficient of determination Rii 0.9905, the gradient equals 7 ∙ ten-four; that is, εb = vii ∙ 10-4. Therefore, with b = 1cm, ε will be 1428.57 M-1.cm-1 (1/vii∙10-4).
References
- Wikipedia. (2018). Tooth attenuation coefficient. Recovered from: en.wikipedia.org
- Science Struck. (2018). Molar Absorptivity. Recovered from: sciencestruck.com
- Colorimetric Analysis: (Beer's law or Spectrophotometric Analysis). Recovered from: chem.ucla.edu
- Kerner North. (s.f.). Experiment II - Solution Color, Absorbance, and Beer'due south Constabulary. Recovered from: umich.edu
- Day, R., & Underwood, A. Quantitative Analytical Chemistry (5th ed.). PEARSON Prentice Hall, p-472.
- Gonzáles M. (November 17, 2010). Absorptivity Recovered from: quimica.laguia2000.com
What Is Molar Absorption Coefficient,
Source: https://warbletoncouncil.org/absortividad-molar-4401
Posted by: thompsoncutdomplad.blogspot.com


0 Response to "What Is Molar Absorption Coefficient"
Post a Comment